Nuclear Physics
Nuclear physics is the branch of science which looks inside atoms. It looks at the nucleus, and what effect different interactions have on it.
Image
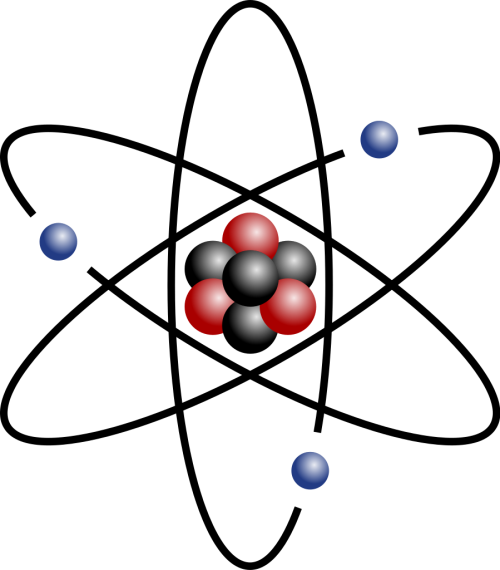
Credit
This work
by Indolences
is licensed under GNU General Public License v2.0 or later
Particle
A particle is the name given to a very small object which has features like size and mass.
All the matter in the Universe is made of particles.
Image
Credit
This work
by The Schools' Observatory
is licensed under All rights reserved
